Adrenal Adenoma2 T1FS on:
[Wikipedia]
[Google]
[Amazon]
The adrenal glands (also known as suprarenal glands) are
 The adrenal glands are located on both sides of the body in the
The adrenal glands are located on both sides of the body in the
 The adrenal cortex is the outer region and also the largest part of an adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones.
The adrenal cortex is the outermost layer of the adrenal gland. Within the cortex are three layers, called "zones". When viewed under a microscope each layer has a distinct appearance, and each has a different function. The adrenal cortex is devoted to production of
The adrenal cortex is the outer region and also the largest part of an adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones.
The adrenal cortex is the outermost layer of the adrenal gland. Within the cortex are three layers, called "zones". When viewed under a microscope each layer has a distinct appearance, and each has a different function. The adrenal cortex is devoted to production of
 The adrenal gland secretes a number of different hormones which are metabolised by enzymes either within the gland or in other parts of the body. These hormones are involved in a number of essential biological functions.
The adrenal gland secretes a number of different hormones which are metabolised by enzymes either within the gland or in other parts of the body. These hormones are involved in a number of essential biological functions.
 All corticosteroid hormones share cholesterol as a common precursor. Therefore, the first step in
All corticosteroid hormones share cholesterol as a common precursor. Therefore, the first step in  Glucocorticoids are under the regulatory influence of the HPA axis, hypothalamus-pituitary-adrenal (HPA) axis. Glucocorticoid synthesis is stimulated by adrenocorticotropic hormone (ACTH), a hormone released into the bloodstream by the anterior pituitary. In turn, production of ACTH is stimulated by the presence of corticotropin-releasing hormone (CRH), which is released by neurons of the hypothalamus. ACTH acts on the adrenal cells first by increasing the levels of StAR within the cells, and then of all steroidogenic P450 enzymes. The HPA axis is an example of a negative feedback system, in which cortisol itself acts as a direct inhibitor of both CRH and ACTH synthesis. The HPA axis also interacts with the immune system through increased secretion of ACTH at the presence of certain molecules of the inflammatory response.
Mineralocorticoid secretion is regulated mainly by the renin–angiotensin–aldosterone system (RAAS), the concentration of potassium, and to a lesser extent the concentration of ACTH. Sensors of blood pressure in the juxtaglomerular apparatus of the kidneys release the enzyme renin into the blood, which starts a cascade of reactions that lead to formation of angiotensin II. Angiotensin receptors in cells of the zona glomerulosa recognize the substance, and upon binding they stimulate the release of aldosterone.
Glucocorticoids are under the regulatory influence of the HPA axis, hypothalamus-pituitary-adrenal (HPA) axis. Glucocorticoid synthesis is stimulated by adrenocorticotropic hormone (ACTH), a hormone released into the bloodstream by the anterior pituitary. In turn, production of ACTH is stimulated by the presence of corticotropin-releasing hormone (CRH), which is released by neurons of the hypothalamus. ACTH acts on the adrenal cells first by increasing the levels of StAR within the cells, and then of all steroidogenic P450 enzymes. The HPA axis is an example of a negative feedback system, in which cortisol itself acts as a direct inhibitor of both CRH and ACTH synthesis. The HPA axis also interacts with the immune system through increased secretion of ACTH at the presence of certain molecules of the inflammatory response.
Mineralocorticoid secretion is regulated mainly by the renin–angiotensin–aldosterone system (RAAS), the concentration of potassium, and to a lesser extent the concentration of ACTH. Sensors of blood pressure in the juxtaglomerular apparatus of the kidneys release the enzyme renin into the blood, which starts a cascade of reactions that lead to formation of angiotensin II. Angiotensin receptors in cells of the zona glomerulosa recognize the substance, and upon binding they stimulate the release of aldosterone.
 Addison's disease refers to primary hypoadrenalism, which is a deficiency in glucocorticoid and mineralocorticoid production by the adrenal gland. In the Western world, Addison's disease is most commonly an autoimmunity, autoimmune condition, in which the body produces Antibody, antibodies against cells of the adrenal cortex. Worldwide, the disease is more frequently caused by infection, especially from tuberculosis. A distinctive feature of Addison's disease is hyperpigmentation of the skin, which presents with other nonspecific symptoms such as fatigue.
A complication seen in untreated Addison's disease and other types of primary adrenal insufficiency is the adrenal crisis, a medical emergency in which low glucocorticoid and mineralocorticoid levels result in hypovolemic shock and symptoms such as vomiting and fever. An adrenal crisis can progressively lead to stupor and coma. The management of adrenal crises includes the application of hydrocortisone injections.
Addison's disease refers to primary hypoadrenalism, which is a deficiency in glucocorticoid and mineralocorticoid production by the adrenal gland. In the Western world, Addison's disease is most commonly an autoimmunity, autoimmune condition, in which the body produces Antibody, antibodies against cells of the adrenal cortex. Worldwide, the disease is more frequently caused by infection, especially from tuberculosis. A distinctive feature of Addison's disease is hyperpigmentation of the skin, which presents with other nonspecific symptoms such as fatigue.
A complication seen in untreated Addison's disease and other types of primary adrenal insufficiency is the adrenal crisis, a medical emergency in which low glucocorticoid and mineralocorticoid levels result in hypovolemic shock and symptoms such as vomiting and fever. An adrenal crisis can progressively lead to stupor and coma. The management of adrenal crises includes the application of hydrocortisone injections.
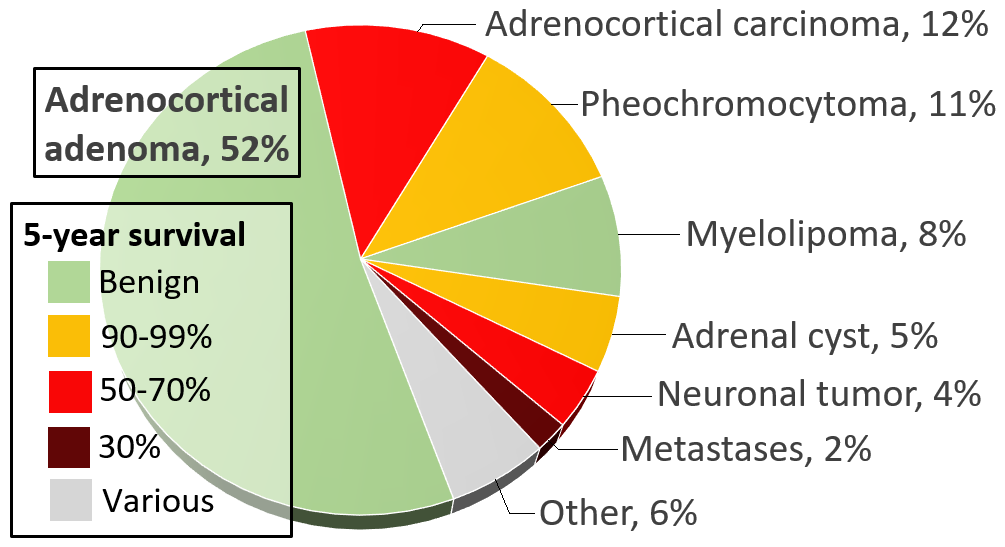 Adrenal tumors are commonly found as incidentalomas, unexpected asymptomatic tumors incidental findings, found during medical imaging. They are seen in around 3.4% of X-ray computed tomography, CT scans, and in most cases they are benign Adrenocortical adenoma, adenomas. Adrenal carcinomas are very rare, with an incidence (epidemiology), incidence of 1 case per million per year.
Pheochromocytomas are tumors of the adrenal medulla that arise from chromaffin cells. They can produce a variety of nonspecific symptoms, which include headaches, sweating, anxiety and palpitations. Common signs include hypertension and tachycardia. Surgery, especially adrenal laparoscopy, is the most common treatment for small pheochromocytomas.
Adrenal tumors are commonly found as incidentalomas, unexpected asymptomatic tumors incidental findings, found during medical imaging. They are seen in around 3.4% of X-ray computed tomography, CT scans, and in most cases they are benign Adrenocortical adenoma, adenomas. Adrenal carcinomas are very rare, with an incidence (epidemiology), incidence of 1 case per million per year.
Pheochromocytomas are tumors of the adrenal medulla that arise from chromaffin cells. They can produce a variety of nonspecific symptoms, which include headaches, sweating, anxiety and palpitations. Common signs include hypertension and tachycardia. Surgery, especially adrenal laparoscopy, is the most common treatment for small pheochromocytomas.
Adrenal gland at the Human Protein Atlas
*
Adrenal gland histology
* – "Adrenal Gland" * * – "Posterior Abdominal Wall: The Retroperitoneal Fat and Suprarenal Glands"
from Colorado State University * {{DEFAULTSORT:Adrenal Gland Adrenal gland, Adrenaline
endocrine gland
Endocrine glands are ductless glands of the endocrine system that secrete their products, hormones, directly into the blood. The major glands of the endocrine system include the pineal gland, pituitary gland, pancreas, ovaries, testes, ...
s that produce a variety of hormones including adrenaline
Adrenaline, also known as epinephrine, is a hormone and medication which is involved in regulating visceral functions (e.g., respiration). It appears as a white microcrystalline granule. Adrenaline is normally produced by the adrenal glands an ...
and the steroids aldosterone and cortisol. They are found above the kidneys
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; bloo ...
. Each gland has an outer cortex
Cortex or cortical may refer to:
Biology
* Cortex (anatomy), the outermost layer of an organ
** Cerebral cortex, the outer layer of the vertebrate cerebrum, part of which is the ''forebrain''
*** Motor cortex, the regions of the cerebral cortex i ...
which produces steroid hormone
A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids (typically made in the adrenal cortex, hence ''cortico-'') and sex steroids (typically made in the gonads or placenta). Withi ...
s and an inner medulla
Medulla or Medullary may refer to:
Science
* Medulla oblongata, a part of the brain stem
* Renal medulla, a part of the kidney
* Adrenal medulla, a part of the adrenal gland
* Medulla of ovary, a stroma in the center of the ovary
* Medulla of t ...
. The adrenal cortex itself is divided into three main zones: the zona glomerulosa
The ''zona glomerulosa'' (sometimes, glomerular zone) of the adrenal gland is the most superficial layer of the adrenal cortex, lying directly beneath the renal capsule. Its cells are ovoid and arranged in clusters or arches (''glomus'' is Latin ...
, the zona fasciculata
The ''zona fasciculata'' (sometimes, fascicular or fasciculate zone) constitutes the middle and also the widest zone of the adrenal cortex, sitting directly beneath the ''zona glomerulosa''. Constituent cells are organized into bundles or "fascicl ...
and the zona reticularis
The zona reticularis (sometimes, reticulate zone) is the innermost layer of the adrenal cortex, lying deep to the zona fasciculata and superficial to the adrenal medulla. The cells are arranged cords that project in different directions giving a ...
.
The adrenal cortex produces three main types of steroid hormone
A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids (typically made in the adrenal cortex, hence ''cortico-'') and sex steroids (typically made in the gonads or placenta). Withi ...
s: mineralocorticoid
Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances ( electrolyte balance and fluid balance). The primary ...
s, glucocorticoid
Glucocorticoids (or, less commonly, glucocorticosteroids) are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every verteb ...
s, and androgen
An androgen (from Greek ''andr-'', the stem of the word meaning "man") is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This in ...
s. Mineralocorticoids (such as aldosterone) produced in the zona glomerulosa help in the regulation of blood pressure and electrolyte balance. The glucocorticoids cortisol and cortisone
Cortisone is a pregnene (21-carbon) steroid hormone. It is a naturally-occurring corticosteroid metabolite that is also used as a pharmaceutical prodrug; it is not synthesized in the adrenal glands. Cortisol is converted by the action of the enz ...
are synthesized in the zona fasciculata; their functions include the regulation of metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run c ...
and immune system
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splint ...
suppression. The innermost layer of the cortex, the zona reticularis, produces androgens that are converted to fully functional sex hormones in the gonads and other target organs. The production of steroid hormones is called steroidogenesis
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and ...
, and involves a number of reactions and processes that take place in cortical cells. The medulla produces the catecholamine
A catecholamine (; abbreviated CA) is a monoamine neurotransmitter, an organic compound that has a catechol (benzene with two hydroxyl side groups next to each other) and a side-chain amine.
Catechol can be either a free molecule or a su ...
s, which function to produce a rapid response throughout the body in stress
Stress may refer to:
Science and medicine
* Stress (biology), an organism's response to a stressor such as an environmental condition
* Stress (linguistics), relative emphasis or prominence given to a syllable in a word, or to a word in a phrase ...
situations.
A number of endocrine disease
Endocrine diseases are disorders of the endocrine system. The branch of medicine associated with endocrine disorders is known as endocrinology.
Types of disease
Broadly speaking, endocrine disorders may be subdivided into three groups:
# Endocrin ...
s involve dysfunctions of the adrenal gland. Overproduction of cortisol leads to Cushing's syndrome
Cushing's syndrome is a collection of signs and symptoms due to prolonged exposure to glucocorticoids such as cortisol. Signs and symptoms may include high blood pressure, abdominal obesity but with thin arms and legs, reddish stretch marks, a ...
, whereas insufficient production is associated with Addison's disease
Addison's disease, also known as primary adrenal insufficiency, is a rare long-term endocrine disorder characterized by inadequate production of the steroid hormones cortisol and aldosterone by the two outer layers of the cells of the adre ...
. Congenital adrenal hyperplasia
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders characterized by impaired cortisol synthesis. It results from the deficiency of one of the five enzymes required for the synthesis of cortisol in the adrenal cort ...
is a genetic disease produced by dysregulation of endocrine control mechanisms. A variety of tumors
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
can arise from adrenal tissue and are commonly found in medical imaging when searching for other diseases.
Structure
 The adrenal glands are located on both sides of the body in the
The adrenal glands are located on both sides of the body in the retroperitoneum
The retroperitoneal space (retroperitoneum) is the anatomical space (sometimes a potential space) behind (''retro'') the peritoneum. It has no specific delineating anatomical structures. Organs are retroperitoneal if they have peritoneum on their ...
, above and slightly medial to the kidney
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blo ...
s. In humans, the right adrenal gland is pyramidal in shape, whereas the left is semilunar or crescent shaped and somewhat larger. The adrenal glands measure approximately 5 cm in length, 3 cm in width, and up to 1 cm in thickness. Their combined weight in an adult human ranges from 7 to 10 grams. The glands are yellowish in colour.
The adrenal glands are surrounded by a fatty capsule and lie within the renal fascia
The renal fascia is a layer of connective tissue encapsulating the kidneys and the adrenal glands. It can be divided into:
*The anterior renal fascia, also called Gerota's fascia (after Dimitrie Gerota)
*The posterior renal fascia, also called Zuc ...
, which also surrounds the kidneys. A weak septum (wall) of connective tissue separates the glands from the kidneys. The adrenal glands are directly below the diaphragm, and are attached to the crura of the diaphragm by the renal fascia.
Each adrenal gland has two distinct parts, each with a unique function, the outer adrenal cortex and the inner medulla
Medulla or Medullary may refer to:
Science
* Medulla oblongata, a part of the brain stem
* Renal medulla, a part of the kidney
* Adrenal medulla, a part of the adrenal gland
* Medulla of ovary, a stroma in the center of the ovary
* Medulla of t ...
, both of which produce hormones.
Adrenal cortex
 The adrenal cortex is the outer region and also the largest part of an adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones.
The adrenal cortex is the outermost layer of the adrenal gland. Within the cortex are three layers, called "zones". When viewed under a microscope each layer has a distinct appearance, and each has a different function. The adrenal cortex is devoted to production of
The adrenal cortex is the outer region and also the largest part of an adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones.
The adrenal cortex is the outermost layer of the adrenal gland. Within the cortex are three layers, called "zones". When viewed under a microscope each layer has a distinct appearance, and each has a different function. The adrenal cortex is devoted to production of hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are require ...
s, namely aldosterone, cortisol, and androgen
An androgen (from Greek ''andr-'', the stem of the word meaning "man") is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This in ...
s.
Zona glomerulosa
The outermost zone of the adrenal cortex is thezona glomerulosa
The ''zona glomerulosa'' (sometimes, glomerular zone) of the adrenal gland is the most superficial layer of the adrenal cortex, lying directly beneath the renal capsule. Its cells are ovoid and arranged in clusters or arches (''glomus'' is Latin ...
. It lies immediately under the fibrous capsule of the gland. Cells in this layer form oval groups, separated by thin strands of connective tissue from the fibrous capsule of the gland and carry wide capillaries
A capillary is a small blood vessel from 5 to 10 micrometres (μm) in diameter. Capillaries are composed of only the tunica intima, consisting of a thin wall of simple squamous endothelial cells. They are the smallest blood vessels in the body: ...
.
This layer is the main site for production of aldosterone, a mineralocorticoid
Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances ( electrolyte balance and fluid balance). The primary ...
, by the action of the enzyme aldosterone synthase
Aldosterone synthase, also called steroid 18-hydroxylase, corticosterone 18-monooxygenase or P450C18, is a steroid hydroxylase cytochrome P450 enzyme involved in the biosynthesis of the mineralocorticoid aldosterone and other steroids. The enzyme ...
. Aldosterone plays an important role in the long-term regulation of blood pressure.
Zona fasciculata
Thezona fasciculata
The ''zona fasciculata'' (sometimes, fascicular or fasciculate zone) constitutes the middle and also the widest zone of the adrenal cortex, sitting directly beneath the ''zona glomerulosa''. Constituent cells are organized into bundles or "fascicl ...
is situated between the zona glomerulosa and zona reticularis. Cells in this layer are responsible for producing glucocorticoid
Glucocorticoids (or, less commonly, glucocorticosteroids) are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every verteb ...
s such as cortisol. It is the largest of the three layers, accounting for nearly 80% of the volume of the cortex. In the zona fasciculata, cells are arranged in columns radially oriented towards the medulla. Cells contain numerous lipid droplets, abundant mitochondria and a complex smooth endoplasmic reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum ...
.
Zona reticularis
The innermost cortical layer, thezona reticularis
The zona reticularis (sometimes, reticulate zone) is the innermost layer of the adrenal cortex, lying deep to the zona fasciculata and superficial to the adrenal medulla. The cells are arranged cords that project in different directions giving a ...
, lies directly adjacent to the medulla. It produces androgen
An androgen (from Greek ''andr-'', the stem of the word meaning "man") is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This in ...
s, mainly dehydroepiandrosterone
Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone precursor. It is one of the most abundant circulating steroids in humans. DHEA is produced in the adrenal glands, the gonads, and the brain. It fun ...
(DHEA), DHEA sulfate
Dehydroepiandrosterone sulfate, abbreviated as DHEA sulfate or DHEA-S, also known as androstenolone sulfate, is an endogenous androstane steroid that is produced by the adrenal cortex. It is the 3β-sulfate ester and a metabolite of dehydroep ...
(DHEA-S), and androstenedione (the precursor to testosterone
Testosterone is the primary sex hormone and anabolic steroid in males. In humans, testosterone plays a key role in the development of male reproductive tissues such as testes and prostate, as well as promoting secondary sexual characteristi ...
) in humans. Its small cells form irregular cords and clusters, separated by capillaries and connective tissue. The cells contain relatively small quantities of cytoplasm and lipid droplets, and sometimes display brown lipofuscin
Lipofuscin is the name given to fine yellow-brown pigment granules composed of lipid-containing residues of lysosomal digestion. It is considered to be one of the aging or "wear-and-tear" pigments, found in the liver, kidney, heart muscle, reti ...
pigment.
Medulla
The adrenal medulla is at the centre of each adrenal gland, and is surrounded by the adrenal cortex. The chromaffin cells of the medulla are the body's main source of thecatecholamine
A catecholamine (; abbreviated CA) is a monoamine neurotransmitter, an organic compound that has a catechol (benzene with two hydroxyl side groups next to each other) and a side-chain amine.
Catechol can be either a free molecule or a su ...
s, such as adrenaline and noradrenaline, released by the medulla. Approximately 20% noradrenaline (norepinephrine) and 80% adrenaline (epinephrine) are secreted here.
The adrenal medulla is driven by the sympathetic nervous system via preganglionic fibers originating in the thoracic spinal cord, from vertebrae T5–T11. Because it is innervated by preganglionic nerve fibers, the adrenal medulla can be considered as a specialized sympathetic ganglion. Unlike other sympathetic ganglia, however, the adrenal medulla lacks distinct synapses and releases its secretions directly into the blood.
Blood supply
The adrenal glands have one of the greatest blood supply rates per gram of tissue of any organ: up to 60 arteriole, small arteries may enter each gland. Three arteries usually supply each adrenal gland: * The superior suprarenal artery, a branch of the inferior phrenic arteries, inferior phrenic artery * The middle suprarenal artery, a direct branch of the abdominal aorta * The inferior suprarenal artery, a branch of the renal artery These blood vessels supply a network of small arteries within the capsule of the adrenal glands. Thin strands of the capsule enter the glands, carrying blood to them. Vein, Venous blood is drained from the glands by the suprarenal veins, usually one for each gland: * The right suprarenal vein drains into the inferior vena cava * The left suprarenal vein drains into the left renal vein or the left inferior phrenic vein. The central adrenomedullary vein, in the adrenal medulla, is an unusual type of blood vessel. Its structure is different from the other veins in that the smooth muscle in its tunica media (the middle layer of the vessel) is arranged in conspicuous, longitudinally oriented bundles.Variability
The adrenal glands may not develop at all, or may be fused in the midline behind the aorta. These are associated with other congenital abnormality, congenital abnormalities, such as failure of the kidneys to develop, or fused kidneys. The gland may develop with a partial or complete absence of the cortex, or may develop in an unusual location.Function
 The adrenal gland secretes a number of different hormones which are metabolised by enzymes either within the gland or in other parts of the body. These hormones are involved in a number of essential biological functions.
The adrenal gland secretes a number of different hormones which are metabolised by enzymes either within the gland or in other parts of the body. These hormones are involved in a number of essential biological functions.
Corticosteroids
Corticosteroids are a group of steroid hormones produced from the cortex of the adrenal gland, from which they are named. * Mineralocorticoids such as aldosterone regulate salt ("mineral") balance and blood pressureMarieb Human Anatomy & Physiology 9th edition, chapter:16, page:629, question number:14 * Glucocorticoids such as cortisol influence metabolism rates of proteins, fats and sugars ("glucose"). *Androgens such asdehydroepiandrosterone
Dehydroepiandrosterone (DHEA), also known as androstenolone, is an endogenous steroid hormone precursor. It is one of the most abundant circulating steroids in humans. DHEA is produced in the adrenal glands, the gonads, and the brain. It fun ...
.
;Mineralocorticoids
The adrenal gland produces aldosterone, a mineralocorticoid
Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances ( electrolyte balance and fluid balance). The primary ...
, which is important in the regulation of salt ("mineral") balance and blood volume. In the kidneys, aldosterone acts on the distal convoluted tubules and the Collecting duct system, collecting ducts by increasing the reabsorption of Sodium in biology, sodium and the excretion of both potassium and hydrogen ions. Aldosterone is responsible for the reabsorption of about 2% of filtered glomerular filtrate. Sodium retention is also a response of the distal colon and sweat glands to aldosterone receptor stimulation. Angiotensin II and extracellular potassium are the two main regulators of aldosterone production. The amount of sodium present in the body affects the extracellular volume, which in turn influences blood pressure. Therefore, the effects of aldosterone in sodium retention are important for the regulation of blood pressure.
;Glucocorticoids
Cortisol is the main glucocorticoid
Glucocorticoids (or, less commonly, glucocorticosteroids) are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every verteb ...
in humans. In species that do not create cortisol, this role is played by corticosterone instead. Glucocorticoids have many effects on metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run c ...
. As their name suggests, they increase the circulating level of glucose. This is the result of an increase in the mobilization of amino acids from protein and the stimulation of Gluconeogenesis, synthesis of glucose from these amino acids in the liver. In addition, they increase the levels of free fatty acids, which cells can use as an alternative to glucose to obtain energy. Glucocorticoids also have effects unrelated to the regulation of blood sugar levels, including the suppression of the immune system and a potent anti-inflammatory effect. Cortisol reduces the capacity of osteoblasts to produce new bone tissue and decreases the absorption of calcium in the Human gastrointestinal tract, gastrointestinal tract.
The adrenal gland secretes a basal level of cortisol but can also produce bursts of the hormone in response to adrenocorticotropic hormone (ACTH) from the anterior pituitary. Cortisol is not evenly released during the day – its concentrations in the blood are highest in the early morning and lowest in the evening as a result of the circadian rhythm of ACTH secretion. Cortisone is an inactive product of the action of the enzyme 11-Beta hydroxysteroid dehydrogenase, 11β-HSD on cortisol. The reaction catalyzed by 11β-HSD is reversible, which means that it can turn administered cortisone into cortisol, the biologically active hormone.
;Formation
steroidogenesis
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and ...
is cholesterol uptake or synthesis. Cells that produce steroid hormones can acquire cholesterol through two paths. The main source is through dietary cholesterol transported via the blood as cholesterol esters within low density lipoproteins (LDL). LDL enters the cells through receptor-mediated endocytosis. The other source of cholesterol is synthesis in the cell's endoplasmic reticulum. Synthesis can compensate when LDL levels are abnormally low. In the lysosome, cholesterol esters are converted to free cholesterol, which is then used for steroidogenesis or stored in the cell.
The initial part of conversion of cholesterol into steroid hormones involves a number of enzymes of the cytochrome P450 family that are located in the inner membrane of mitochondria. Transport of cholesterol from the outer to the inner membrane is facilitated by steroidogenic acute regulatory protein and is the rate-limiting step of steroid synthesis.
The layers of the adrenal gland differ by function, with each layer having distinct enzymes that produce different hormones from a common precursor. The first enzymatic step in the production of all steroid hormones is cleavage of the cholesterol side chain, a reaction that forms pregnenolone as a product and is catalyzed by the enzyme P450scc, also known as ''cholesterol desmolase''. After the production of pregnenolone, specific enzymes of each cortical layer further modify it. Enzymes involved in this process include both mitochondrial and Microsome, microsomal P450s and hydroxysteroid dehydrogenases. Usually a number of intermediate steps in which pregnenolone is modified several times are required to form the functional hormones. Enzymes that catalyze reactions in these metabolic pathways are involved in a number of endocrine diseases. For example, the most common form of congenital adrenal hyperplasia develops as a result of deficiency of 21-hydroxylase, an enzyme involved in an intermediate step of cortisol production.
;Regulation
Androgens
Cells inzona reticularis
The zona reticularis (sometimes, reticulate zone) is the innermost layer of the adrenal cortex, lying deep to the zona fasciculata and superficial to the adrenal medulla. The cells are arranged cords that project in different directions giving a ...
of the adrenal glands produce male sex hormones, or androgen
An androgen (from Greek ''andr-'', the stem of the word meaning "man") is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This in ...
s, the most important of which is Dehydroepiandrosterone, DHEA. In general, these hormones do not have an overall effect in the male body, and are converted to more potent androgens such as testosterone
Testosterone is the primary sex hormone and anabolic steroid in males. In humans, testosterone plays a key role in the development of male reproductive tissues such as testes and prostate, as well as promoting secondary sexual characteristi ...
and Dihydrotestosterone, DHT or to estrogens (female sex hormones) in the gonads, acting in this way as a metabolic intermediate.
Catecholamines
Primarily referred to in the United States as epinephrine and norepinephrine,adrenaline
Adrenaline, also known as epinephrine, is a hormone and medication which is involved in regulating visceral functions (e.g., respiration). It appears as a white microcrystalline granule. Adrenaline is normally produced by the adrenal glands an ...
and noradrenaline are catecholamine
A catecholamine (; abbreviated CA) is a monoamine neurotransmitter, an organic compound that has a catechol (benzene with two hydroxyl side groups next to each other) and a side-chain amine.
Catechol can be either a free molecule or a su ...
s, water-soluble organic compound, compounds that have a structure made of a catechol group and an amine group. The adrenal glands are responsible for most of the adrenaline that circulates in the body, but only for a small amount of circulating noradrenaline. These hormones are released by the adrenal medulla, which contains a dense network of blood vessels. Adrenaline and noradrenaline act by interacting with adrenoreceptors throughout the body, with effects that include an increase in blood pressure and heart rate. Actions of adrenaline and noradrenaline are responsible for the fight or flight response, characterised by a quickening of breathing and heart rate, an increase in blood pressure, and constriction of blood vessels in many parts of the body.
;Formation
Catecholamines are produced in chromaffin cells in the medulla of the adrenal gland, from tyrosine, a non-essential amino acid derived from food or produced from phenylalanine in the liver. The enzyme tyrosine hydroxylase converts tyrosine to L-DOPA in the first step of catecholamine synthesis. L-DOPA is then converted to dopamine before it can be turned into noradrenaline. In the cytosol, noradrenaline is converted to epinephrine by the enzyme phenylethanolamine N-methyltransferase (PNMT) and stored in granules. Glucocorticoids produced in the adrenal cortex stimulate the synthesis of catecholamines by increasing the levels of tyrosine hydroxylase and PNMT.
Catecholamine release is stimulated by the activation of the sympathetic nervous system. Splanchnic nerves of the sympathetic nervous system innervate the medulla of the adrenal gland. When activated, it evokes the release of catecholamines from the storage granules by stimulating the opening of calcium channels in the cell membrane.
Gene and protein expression
The human genome includes approximately 20,000 protein coding genes and 70% of these Gene expression, genes are expressed in the normal adult adrenal glands. Only some 250 genes are more specifically expressed in the adrenal glands compared to other organs and tissues. The adrenal-gland-specific genes with the highest level of expression include members of the cytochrome P450 superfamily of enzymes. Corresponding proteins are expressed in the different compartments of the adrenal gland, such as CYP11A1, HSD3B2 and FDX1 involved insteroid hormone
A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids (typically made in the adrenal cortex, hence ''cortico-'') and sex steroids (typically made in the gonads or placenta). Withi ...
synthesis and expressed in cortical cell layers, and Phenylethanolamine N-methyltransferase, PNMT and Dopamine beta-hydroxylase, DBH involved in Norepinephrine, noradrenaline and Epinephrine, adrenaline synthesis and expressed in the medulla.
Development
The adrenal glands are composed of two heterogenous types of tissue. In the center is the adrenal medulla, which producesadrenaline
Adrenaline, also known as epinephrine, is a hormone and medication which is involved in regulating visceral functions (e.g., respiration). It appears as a white microcrystalline granule. Adrenaline is normally produced by the adrenal glands an ...
and noradrenaline and releases them into the bloodstream, as part of the sympathetic nervous system. Surrounding the medulla is the cortex
Cortex or cortical may refer to:
Biology
* Cortex (anatomy), the outermost layer of an organ
** Cerebral cortex, the outer layer of the vertebrate cerebrum, part of which is the ''forebrain''
*** Motor cortex, the regions of the cerebral cortex i ...
, which produces a variety of steroid hormone
A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids (typically made in the adrenal cortex, hence ''cortico-'') and sex steroids (typically made in the gonads or placenta). Withi ...
s. These tissues come from different Human embryogenesis, embryological precursors and have distinct prenatal development paths. The cortex of the adrenal gland is derived from mesoderm, whereas the medulla is derived from the neural crest, which is of ectodermal origin.
The adrenal glands in a newborn baby are much larger as a proportion of the body size than in an adult. For example, at age three months the glands are four times the size of the kidneys. The size of the glands decreases relatively after birth, mainly because of shrinkage of the cortex. The cortex, which almost completely disappears by age 1, develops again from age 4–5. The glands weigh about 1 g at birth and develop to an adult weight of about 4 grams each. In a fetus the glands are first detectable after the sixth week of development.
Cortex
Adrenal cortex tissue is derived from the intermediate mesoderm. It first appears 33 days after fertilisation, shows Steroid#Steroidogenesis, steroid hormone production capabilities by the eighth week and undergoes rapid growth during the first trimester of pregnancy. The fetal adrenal cortex is different from its adult counterpart, as it is composed of two distinct zones: the inner "fetal" zone, which carries most of the hormone-producing activity, and the outer "definitive" zone, which is in a cell proliferation, proliferative phase. The fetal zone produces large amounts of adrenalandrogen
An androgen (from Greek ''andr-'', the stem of the word meaning "man") is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This in ...
s (male sex hormones) that are used by the placenta for estrogen biosynthesis. Cortical development of the adrenal gland is regulated mostly by Adrenocorticotropic hormone, ACTH, a hormone produced by the pituitary gland that stimulates cortisol synthesis. During midgestation, the fetal zone occupies most of the cortical volume and produces 100–200 mg/day of DHEA-S, an androgen
An androgen (from Greek ''andr-'', the stem of the word meaning "man") is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This in ...
and precursor of both androgens and estrogens (female sex hormones). Adrenal hormones, especially glucocorticoid
Glucocorticoids (or, less commonly, glucocorticosteroids) are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every verteb ...
s such as cortisol, are essential for prenatal development of organs, particularly for the maturation of the lungs. The adrenal gland decreases in size after birth because of the rapid disappearance of the fetal zone, with a corresponding decrease in androgen secretion.
Adrenarche
During early childhood androgen synthesis and secretion remain low, but several years before puberty (from 6–8 years of age) changes occur in both anatomical and functional aspects of cortical androgen production that lead to increased secretion of the steroids dehydroepiandrosterone, DHEA and dehydroepiandrosterone sulfate, DHEA-S. These changes are part of a process called adrenarche, which has only been described in humans and some other primates. Adrenarche is independent of Adrenocorticotropic hormone, ACTH or gonadotropins and correlates with a progressive thickening of thezona reticularis
The zona reticularis (sometimes, reticulate zone) is the innermost layer of the adrenal cortex, lying deep to the zona fasciculata and superficial to the adrenal medulla. The cells are arranged cords that project in different directions giving a ...
layer of the cortex. Functionally, adrenarche provides a source of androgens for the development of axillary and pubic hair before the beginning of puberty.
Medulla
The adrenal medulla is derived from neural crest, neural crest cells, which come from the ectoderm layer of the embryo. These cells cell migration, migrate from their initial position and aggregate in the vicinity of the dorsal aorta, a primitive blood vessel, which activates the differentiation of these cells through the release of proteins known as Bone morphogenetic protein, BMPs. These cells then undergo a second migration from the dorsal aorta to form the adrenal medulla and other organs of the sympathetic nervous system. Cells of the adrenal medulla are called chromaffin cells because they contain granules that stain with chromium salts, a characteristic not present in all sympathetic organs. Glucocorticoids produced in the adrenal cortex were once thought to be responsible for the differentiation of chromaffin cells. More recent research suggests that Bone morphogenetic protein 4, BMP-4 secreted in adrenal tissue is the main responsible for this, and that glucocorticoids only play a role in the subsequent development of the cells.Clinical significance
The normal function of the adrenal gland may be impaired by conditions such as infections, tumors, genetic disorders and autoimmune diseases, or as a side effect of medical therapy. These disorders affect the gland either directly (as with infections or autoimmune diseases) or as a result of the dysregulation of hormone production (as in some types ofCushing's syndrome
Cushing's syndrome is a collection of signs and symptoms due to prolonged exposure to glucocorticoids such as cortisol. Signs and symptoms may include high blood pressure, abdominal obesity but with thin arms and legs, reddish stretch marks, a ...
) leading to an excess or insufficiency of adrenal hormones and the related symptoms.
Corticosteroid overproduction
Cushing's syndrome
Cushing's syndrome
Cushing's syndrome is a collection of signs and symptoms due to prolonged exposure to glucocorticoids such as cortisol. Signs and symptoms may include high blood pressure, abdominal obesity but with thin arms and legs, reddish stretch marks, a ...
is the manifestation of glucocorticoid excess. It can be the result of a prolonged treatment with glucocorticoids or be caused by an underlying disease which produces alterations in the HPA axis or the production of cortisol. Causes can be further classified into Adrenocorticotropic hormone, ACTH-dependent or ACTH-independent. The most common cause of endogeny, endogenous Cushing's syndrome is a pituitary adenoma which causes an excessive production of ACTH. The disease produces a wide variety of signs and symptoms which include obesity, diabetes, increased blood pressure, excessive body hair (hirsutism), osteoporosis, depression, and most distinctively, striae, stretch marks in the skin, caused by its progressive thinning.
Primary aldosteronism
When the zona glomerulosa produces excess aldosterone, the result is primary aldosteronism. Causes for this condition are bilateral hyperplasia (excessive tissue growth) of the glands, or aldosterone-producing adenomas (a condition called Conn's syndrome). Primary aldosteronism produces hypertension and electrolyte imbalance, increasing potassium depletion sodium retention.Adrenal insufficiency
Adrenal insufficiency (the deficiency ofglucocorticoid
Glucocorticoids (or, less commonly, glucocorticosteroids) are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every verteb ...
s) occurs in about 5 in 10,000 in the general population. Diseases classified as ''primary adrenal insufficiency'' (including Addison's disease
Addison's disease, also known as primary adrenal insufficiency, is a rare long-term endocrine disorder characterized by inadequate production of the steroid hormones cortisol and aldosterone by the two outer layers of the cells of the adre ...
and genetic causes) directly affect the adrenal cortex. If a problem that affects the hypothalamic-pituitary-adrenal axis arises outside the gland, it is a ''secondary adrenal insufficiency''.
Addison's disease
 Addison's disease refers to primary hypoadrenalism, which is a deficiency in glucocorticoid and mineralocorticoid production by the adrenal gland. In the Western world, Addison's disease is most commonly an autoimmunity, autoimmune condition, in which the body produces Antibody, antibodies against cells of the adrenal cortex. Worldwide, the disease is more frequently caused by infection, especially from tuberculosis. A distinctive feature of Addison's disease is hyperpigmentation of the skin, which presents with other nonspecific symptoms such as fatigue.
A complication seen in untreated Addison's disease and other types of primary adrenal insufficiency is the adrenal crisis, a medical emergency in which low glucocorticoid and mineralocorticoid levels result in hypovolemic shock and symptoms such as vomiting and fever. An adrenal crisis can progressively lead to stupor and coma. The management of adrenal crises includes the application of hydrocortisone injections.
Addison's disease refers to primary hypoadrenalism, which is a deficiency in glucocorticoid and mineralocorticoid production by the adrenal gland. In the Western world, Addison's disease is most commonly an autoimmunity, autoimmune condition, in which the body produces Antibody, antibodies against cells of the adrenal cortex. Worldwide, the disease is more frequently caused by infection, especially from tuberculosis. A distinctive feature of Addison's disease is hyperpigmentation of the skin, which presents with other nonspecific symptoms such as fatigue.
A complication seen in untreated Addison's disease and other types of primary adrenal insufficiency is the adrenal crisis, a medical emergency in which low glucocorticoid and mineralocorticoid levels result in hypovolemic shock and symptoms such as vomiting and fever. An adrenal crisis can progressively lead to stupor and coma. The management of adrenal crises includes the application of hydrocortisone injections.
Secondary adrenal insufficiency
In secondary adrenal insufficiency, a dysfunction of the hypothalamic-pituitary-adrenal axis leads to decreased stimulation of the adrenal cortex. Apart from suppression of the axis by glucocorticoid therapy, the most common cause of secondary adrenal insufficiency are tumors that affect the production of adrenocorticotropic hormone (ACTH) by the pituitary gland. This type of adrenal insufficiency usually does not affect the production ofmineralocorticoid
Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances ( electrolyte balance and fluid balance). The primary ...
s, which are under regulation of the renin–angiotensin system instead.
Congenital adrenal hyperplasia
Congenital adrenal hyperplasia
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders characterized by impaired cortisol synthesis. It results from the deficiency of one of the five enzymes required for the synthesis of cortisol in the adrenal cort ...
is a congenital disease in which mutations of enzymes that produce steroid hormones result in a glucocorticoid deficiency and malfunction of the negative feedback loop of the HPA axis. In the HPA axis, cortisol (a glucocorticoid) inhibits the release of corticotropin-releasing hormone, CRH and Adrenocorticotropic hormone, ACTH, hormones that in turn stimulate corticosteroid synthesis. As cortisol cannot be synthesized, these hormones are released in high quantities and stimulate production of other adrenal steroids instead. The most common form of congenital adrenal hyperplasia is due to 21-hydroxylase deficiency. 21-hydroxylase is necessary for production of both mineralocorticoids and glucocorticoids, but not androgen
An androgen (from Greek ''andr-'', the stem of the word meaning "man") is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This in ...
s. Therefore, ACTH stimulation of the adrenal cortex induces the release of excessive amounts of adrenal androgens, which can lead to the development of ambiguous genitalia and secondary sex characteristics.
Adrenal tumors
 Adrenal tumors are commonly found as incidentalomas, unexpected asymptomatic tumors incidental findings, found during medical imaging. They are seen in around 3.4% of X-ray computed tomography, CT scans, and in most cases they are benign Adrenocortical adenoma, adenomas. Adrenal carcinomas are very rare, with an incidence (epidemiology), incidence of 1 case per million per year.
Pheochromocytomas are tumors of the adrenal medulla that arise from chromaffin cells. They can produce a variety of nonspecific symptoms, which include headaches, sweating, anxiety and palpitations. Common signs include hypertension and tachycardia. Surgery, especially adrenal laparoscopy, is the most common treatment for small pheochromocytomas.
Adrenal tumors are commonly found as incidentalomas, unexpected asymptomatic tumors incidental findings, found during medical imaging. They are seen in around 3.4% of X-ray computed tomography, CT scans, and in most cases they are benign Adrenocortical adenoma, adenomas. Adrenal carcinomas are very rare, with an incidence (epidemiology), incidence of 1 case per million per year.
Pheochromocytomas are tumors of the adrenal medulla that arise from chromaffin cells. They can produce a variety of nonspecific symptoms, which include headaches, sweating, anxiety and palpitations. Common signs include hypertension and tachycardia. Surgery, especially adrenal laparoscopy, is the most common treatment for small pheochromocytomas.
History
Bartolomeo Eustachi, an Italian anatomist, is credited with the first description of the adrenal glands in 1563–4. However, these publications were part of the papal library and did not receive public attention, which was first received with Caspar Bartholin the Elder's illustrations in 1611. The adrenal glands are named for their location relative to the kidneys. The term "adrenal" comes from Latin ''wikt:ad-, ad'', "near", and '':wikt:ren, ren'', "kidney". Similarly, "suprarenal", as termed by Jean Riolan the Younger in 1629, is derived from the Latin '':wikt:supra-, supra'', "above", and ''ren'', "kidney", as well. The suprarenal nature of the glands was not truly accepted until the 19th century, as anatomists clarified the ductless nature of the glands and their likely secretory role – prior to this, there was some debate as to whether the glands were indeed suprarenal or part of the kidney. One of the most recognized works on the adrenal glands came in 1855 with the publication of ''On the Constitutional and Local Effects of Disease of the Suprarenal Capsule'', by the English physician Thomas Addison. In his monography, Addison described what the French physician Georges Phillipe Trousseau, George Trousseau would later nameAddison's disease
Addison's disease, also known as primary adrenal insufficiency, is a rare long-term endocrine disorder characterized by inadequate production of the steroid hormones cortisol and aldosterone by the two outer layers of the cells of the adre ...
, an eponym still used today for a condition of adrenal insufficiency and its related clinical manifestations. In 1894, English physiologists George Oliver (physician), George Oliver and Edward Albert Sharpey-Schafer, Edward Schafer studied the action of adrenal extracts and observed their antihypotensive agent, pressor effects. In the following decades several physicians experimented with extracts from the adrenal cortex to treat Addison's disease. Edward Calvin Kendall, Philip Hench and Tadeusz Reichstein were then awarded the 1950 Nobel Prize in Physiology or Medicine for their discoveries on the structure and effects of the adrenal hormones.
See also
* AdrenopauseReferences
External links
Adrenal gland at the Human Protein Atlas
*
Adrenal gland histology
* – "Adrenal Gland" * * – "Posterior Abdominal Wall: The Retroperitoneal Fat and Suprarenal Glands"
from Colorado State University * {{DEFAULTSORT:Adrenal Gland Adrenal gland, Adrenaline